1. A neutron travelling with a velocity $$v$$ and kinetic energy $$E$$ has a perfectly elastic head-on collision with a nucleus of an atom of mass number $$A$$ at rest. The fraction of total energy retained by the neutron is approximately
A.
$${\left[ {\frac{{\left( {A - 1} \right)}}{{\left( {A + 1} \right)}}} \right]^2}$$
B.
$${\left[ {\frac{{\left( {A + 1} \right)}}{{\left( {A - 1} \right)}}} \right]^2}$$
C.
$${\left[ {\frac{{\left( {A - 1} \right)}}{A}} \right]^2}$$
D.
$${\left[ {\frac{{\left( {A + 1} \right)}}{A}} \right]^2}$$
Answer :
$${\left[ {\frac{{\left( {A - 1} \right)}}{{\left( {A + 1} \right)}}} \right]^2}$$
2. The third line of the Balmer series spectrum of a hydrogen like ion of atomic number $$Z$$ equals to $$108.5\,nm.$$ Then $$Z$$ is
A.
2
B.
5
C.
3
D.
6
Answer :
2
3.
An $$\alpha $$ particle passes rapidly through the exact centre of a hydrogen molecule, moving on a line perpendicular to the inter-nuclear axis. The distance between the nuclei is $$b.$$ Where on its path does the $$\alpha $$ particle experience the greatest force? (Assume that the nuclei do not move much during the passage of the $$\alpha $$ particle. Also neglect the electric field of the electrons in the molecule.)
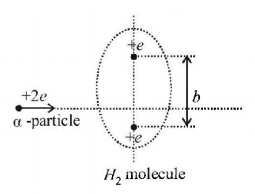
A.
$$\frac{b}{2}$$
B.
$$\frac{b}{{2\sqrt 2 }}$$
C.
$$\frac{b}{{\sqrt 2 }}$$
D.
None of these
Answer :
$$\frac{b}{{2\sqrt 2 }}$$
4. The acceleration of an electron in the first orbit of the hydrogen atom $$\left( {z = 1} \right)$$ is :
A.
$$\frac{{{h^2}}}{{{\pi ^2}{m^2}{r^3}}}$$
B.
$$\frac{{{h^2}}}{{8{\pi ^2}{m^2}{r^3}}}$$
C.
$$\frac{{{h^2}}}{{4{\pi ^2}{m^2}{r^3}}}$$
D.
$$\frac{{{h^2}}}{{4\pi {m^2}{r^3}}}$$
Answer :
$$\frac{{{h^2}}}{{4{\pi ^2}{m^2}{r^3}}}$$
5. Taking the wavelength of first Balmer line in hydrogen spectrum ($$n = 3$$ to $$n = 2$$ ) as $$660\,nm,$$ the wavelength of the $${2^{nd}}$$ Balmer line ($$n = 4$$ to $$n = 2$$ ) will be;
A.
$$889.2\,nm$$
B.
$$488.9\,nm$$
C.
$$642.7\,nm$$
D.
$$388.9\,nm$$
Answer :
$$488.9\,nm$$
6. Electrons are bombarded to excite hydrogen atoms and six spectral lines are observed. If $${E_g}$$ is the ground state energy of hydrogen, the minimum energy the bombarding electrons should posses is
A.
$$\frac{{8{E_g}}}{9}$$
B.
$$\frac{{15{E_g}}}{16}$$
C.
$$\frac{{35{E_g}}}{36}$$
D.
$$\frac{{48{E_g}}}{49}$$
Answer :
$$\frac{{15{E_g}}}{16}$$
7.
The ionization energy of a hydrogen-like Bohr atom is $$4$$ Rydbergs. Find the wavelength of radiation emitted when the electron jumps from the first excited state to the ground state:
$$[1\,Rydberg = 2.2 \times {10^{ - 18}},h = 6.6 \times {10^{ - 34}}Js,c = 3 \times {10^8}m/s.$$ Bohr radius of hydrogen atom $$ = 5 \times {10^{ - 11}}m$$ ]
A.
$$400\,\mathop {\text{A}}\limits^ \circ $$
B.
$$300\,\mathop {\text{A}}\limits^ \circ $$
C.
$$500\,\mathop {\text{A}}\limits^ \circ $$
D.
$$600\,\mathop {\text{A}}\limits^ \circ $$
Answer :
$$300\,\mathop {\text{A}}\limits^ \circ $$
8. The energy difference between the first two levels of hydrogen atom is $$10.2\,eV$$ for another element of atomic number 10 and mass number 20, this will be
A.
$$2040\,eV$$
B.
$$0.201\,eV$$
C.
$$510\,eV$$
D.
$$1020\,eV$$
Answer :
$$1020\,eV$$
9. In a Rutherford scattering experiment when a projectile of charge $${Z_1}$$ and mass $${M_1}$$ approaches a target nucleus of charge $${Z_2}$$ and mass $${M_2},$$ the distance of closest approach is $${r_0}.$$ The energy of the projectile is
A.
directly proportional to $${M_1} \times {M_2}$$
B.
directly proportional to $${Z_1}{Z_2}$$
C.
inversely proportional to $${Z_1}$$
D.
directly proportional to mass $${M_1}$$
Answer :
directly proportional to $${Z_1}{Z_2}$$
10. An energy of $$24.6\,eV$$ is required to remove one of the electrons from a neutral helium atom. The energy in $$\left( {eV} \right)$$ required to remove both the electrons from a neutral helium atom is
A.
38.2
B.
49.2
C.
51.8
D.
79.0
Answer :
79.0