1. An engine has an efficiency of $$\frac{1}{6}.$$ When the temperature of sink is reduced by $${62^ \circ }C,$$ its efficiency is doubled. Temperature of the source is
A.
$${124^ \circ }C$$
B.
$${37^ \circ }C$$
C.
$${62^ \circ }C$$
D.
$${99^ \circ }C$$
Answer :
$${99^ \circ }C$$
2. If the energy input to a Carnot engine is thrice the work it performs then, the fraction of energy rejected to the sink is
A.
$$\frac{1}{3}$$
B.
$$\frac{1}{4}$$
C.
$$\frac{2}{5}$$
D.
$$\frac{2}{3}$$
Answer :
$$\frac{2}{3}$$
3.
A thermodynamic system undergoes cyclic process $$ABCDA$$ as shown in fig. The work done by the system in the cycle is
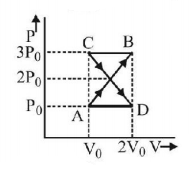
A.
$${P_0}{V_0}$$
B.
$$2{P_0}{V_0}$$
C.
$$\frac{{{P_0}{V_0}}}{2}$$
D.
Zero
Answer :
Zero
4. On a new scale of temperature (which is linear) and called the $$W$$ scale, the freezing and boiling points of water are $${39^ \circ }W$$ and $${239^ \circ }W$$ respectively. What will be the temperature on the new scale, corresponding to a temperature of $${39^ \circ }C$$ on the celsius scale ?
A.
$${78^ \circ }W$$
B.
$${117^ \circ }W$$
C.
$${200^ \circ }W$$
D.
$${139^ \circ }W$$
Answer :
$${117^ \circ }W$$
5.
A thermodynamic system is taken through the cycle $$ABCD$$ as shown in figure. Heat rejected by the gas during the cycle is
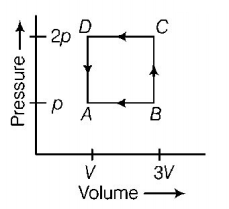
A.
$$2\,pV$$
B.
$$4\,pV$$
C.
$$\frac{1}{2}pV$$
D.
$$pV$$
Answer :
$$2\,pV$$
6. A thermally insulated vessel contains an ideal gas of molecular mass $$M$$ and ratio of specific heats $$\gamma .$$ It is moving with speed $$v$$ and it's suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases by :
A.
$$\frac{{\left( {\gamma - 1} \right)}}{{2\gamma R}}M{v^2}K$$
B.
$$\frac{{\gamma {M^2}v}}{{2R}}K$$
C.
$$\frac{{\left( {\gamma - 1} \right)}}{{2R}}M{v^2}K$$
D.
$$\frac{{\left( {\gamma - 1} \right)}}{{2\left( {\gamma + 1} \right)R}}M{v^2}K$$
Answer :
$$\frac{{\left( {\gamma - 1} \right)}}{{2R}}M{v^2}K$$
7. In a given process on an ideal gas, $$dW = 0$$ and $$dQ < 0.$$ Then for the gas
A.
the temperature will decrease
B.
the volume will increase
C.
the pressure will remain constant
D.
the temperature will increase
Answer :
the temperature will decrease
8. $$100\,g$$ of water is heated from $${30^ \circ }C$$ to $${50^ \circ }C.$$ Ignoring the slight expansion of the water, the change in its internal energy is (specific heat of water is $$4184\,J/kg/K$$ ) :
A.
8.4 $$k J$$
B.
84 $$k J$$
C.
2.1 $$k J$$
D.
4.2 $$k J$$
Answer :
8.4 $$k J$$
9. One mole of an ideal gas goes from an initial state $$A$$ to final state $$B$$ via two processes. It first undergoes isothermal expansion from volume $$V$$ to $$3V$$ and then its volume is reduced from $$3V$$ to $$V$$ at constant pressure. The correct $$p-V$$ diagram representing the two processes is
A.
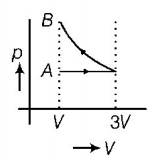

B.
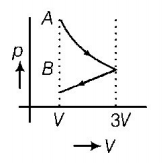

C.
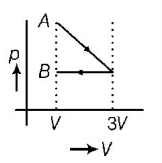

D.
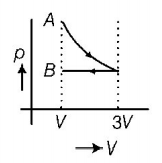

Answer :


10. A Carnot engine works between a source and a sink maintained at constant temperatures $${T_1}$$ and $${T_2}.$$ For efficiency to be the greatest
A.
$${T_1}$$ and $${T_2}$$ should be high
B.
$${T_1}$$ and $${T_2}$$ should be low
C.
$${T_1}$$ should be low and $${T_2}$$ should be high
D.
$${T_1}$$ should be high and $${T_2}$$ should be low
Answer :
$${T_1}$$ should be high and $${T_2}$$ should be low