1. The average translational kinetic energy of $${O_2}$$ (relative molar mass 32) molecules at a particular temperature is $$0.048\,eV.$$ The translational kinetic energy of $${N_2}$$ (relative molar mass 28) molecules in $$eV$$ at the same temperature is
A.
0.0015
B.
0.003
C.
0.048
D.
0.768
Answer :
0.048
2.
Pressure versus temperature graph of an ideal gas of equal number of moles of different volumes are plotted as shown in figure. Choose the correct alternative
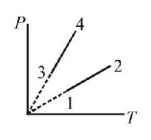
A.
$${V_1} = {V_2},{V_3} = {V_4}\,{\text{and}}\,{V_2} > {V_3}$$
B.
$${V_1} = {V_2},{V_3} = {V_4}\,{\text{and}}\,{V_2} < {V_3}$$
C.
$${V_1} = {V_2} = {V_3} = {V_4}$$
D.
$${V_4} > {V_3} > {V_2} > {V_1}$$
Answer :
$${V_1} = {V_2},{V_3} = {V_4}\,{\text{and}}\,{V_2} > {V_3}$$
3. Two vessels separately contain two ideal gases $$A$$ and $$B$$ at the same temperature, the pressure of $$A$$ being twice that of $$B.$$ Under such conditions, the density of $$A$$ is found to be 1.5 times the density of $$B.$$ The ratio of molecular weight of $$A$$ and $$B$$ is
A.
$$\frac{2}{3}$$
B.
$$\frac{3}{4}$$
C.
$$2$$
D.
$$\frac{1}{2}$$
Answer :
$$\frac{3}{4}$$
4. At very high temperatures vibrational degrees also becomes active. At such temperatures an ideal diatomic gas has a molar specific heat at constant pressure, $${C_p}$$ is
A.
$$\frac{{3R}}{2}$$
B.
$$\frac{{5R}}{2}$$
C.
$$\frac{{6R}}{2}$$
D.
$$\frac{{9R}}{2}$$
Answer :
$$\frac{{9R}}{2}$$
5. Cooking gas containers are kept in a lorry moving with uniform speed. The temperature of the gas molecules inside will
A.
increase
B.
decrease
C.
remain same
D.
decrease for some, while increase for others
Answer :
remain same
6. A perfect gas at $${27^ \circ }C$$ is heated at constant pressure so as to double its volume. The final temperature of the gas will be, close to
A.
$${327^ \circ }C$$
B.
$${200^ \circ }C$$
C.
$${54^ \circ }C$$
D.
$${300^ \circ }C$$
Answer :
$${327^ \circ }C$$
7. $${H_2},{O_2},{N_2}$$ and $$He$$ are enclosed in identical containers under the similar conditions of pressure and temperature. The gases will have
A.
same $$R.M.S.$$ speed
B.
same $$\frac{{K.E.}}{{kg}}$$
C.
different $$\frac{{K.E.}}{{mole}}$$
D.
same $$\frac{{K.E.}}{{vol}}$$
Answer :
same $$\frac{{K.E.}}{{vol}}$$
8. Three closed vessels $$A,B$$ and $$C$$ are at the same temperature $$T$$ and contain gases which obey the Max wellian distribution of velocities. Vessel $$A$$ contain only $${O_2},B$$ only $${N_2}$$ and $$C$$ a mixture of equal quantities of $${O_2}$$ and $${N_2}.$$ If the average speed of the $${O_2}$$ molecules in vessel $$A$$ is $${v_1}$$ that of the $${N_2}$$ molecules in vessel $$B$$ is $${v_2},$$ the average speed of the $${O_2}$$ molecules in vessel $$C$$ is
A.
$$\frac{{{v_1} + {v_2}}}{2}$$
B.
$${{v_1}}$$
C.
$${\left( {{v_1} \cdot {v_2}} \right)^{\frac{1}{2}}}$$
D.
$$\sqrt {\frac{{3kT}}{M}} $$
Answer :
$${{v_1}}$$
9. At constant volume temperature is increased, then
A.
collision on walls will be less
B.
number of collisions per unit time will increase
C.
collisions will be in straight lines
D.
collisions will not change
Answer :
number of collisions per unit time will increase
10. The molecules of a given mass of a gas have $$r.m.s.$$ velocity of $$200\,m{s^{ - 1}}$$ at $${27^ \circ }C$$ and $$1.0 \times {10^5}\,N{m^{ - 2}}$$ pressure. When the temperature and pressure of the gas are respectively, $${127^ \circ }C$$ and $$0.05 \times {10^5}\,N{m^{ - 2}},$$ the $$r.m.s.$$ velocity of its molecules in $$m{s^{ - 1}}$$ is:
A.
$$100\sqrt 2 $$
B.
$$\frac{{400}}{{\sqrt 3 }}$$
C.
$$\frac{{100\sqrt 2 }}{3}$$
D.
$$\frac{{100}}{3}$$
Answer :
$$\frac{{400}}{{\sqrt 3 }}$$