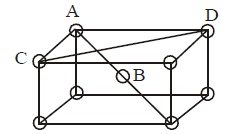
1. Non stoichiometric defects are formed by
A.
$$s$$ - block elements
B.
$$p$$ -block elements
C.
either $$s$$ - block elements or $$d$$ - block elements
D.
only $$d$$ - block elements.
Answer :
only $$d$$ - block elements.
2. $$AB;$$ crystallizes in a body centred cubic lattice with edge length $$'a'$$ equal to $$387\,pm.$$ The distance between two oppositely charged ions in the lattice is
A.
335$$\,pm$$
B.
250$$\,pm$$
C.
200$$\,pm$$
D.
300$$\,pm$$
Answer :
335$$\,pm$$
3. Which of the following arrangements shows schematic alignment of magnetic moments of antiferromagnetic substances ?
A.
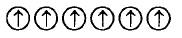

B.
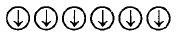

C.
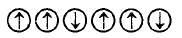

D.
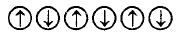

Answer :


4. Solid $$X$$ is a very hard solid which is electrical insulator in solid as well as in molten state and has extremely high melting point. What type of solid is it ?
A.
Ionic solid
B.
Covalent solid
C.
Metallic solid
D.
Molecular solid
Answer :
Covalent solid
5. The edge length of unit cell of a metal having molecular weight $$75\,g/mol$$ is $$5\mathop {\text{A}}\limits^{\text{o}} $$ which crystallizes in cubic lattice. If the density is $$2g/cc$$ then find the radius of metal $$atom.\left( {{N_A} = 6 \times {{10}^{23}}} \right).$$ Given the answer in $$pm.$$
A.
217$$\,pm$$
B.
210$$\,pm$$
C.
220$$\,pm$$
D.
205$$\,pm$$
Answer :
217$$\,pm$$
6. $$CsBr$$ crystallises in a body centered cubic lattice. The unit cell length is $$436.6\,pm.$$ Given that the atomic mass of $$Cs = 133$$ and that of $$Br = 80\,amu$$ and Avogadro number being $$6.02 \times {10^{23}}\,mo{l^{ - 1}},$$ the density of $$CsBr$$ is
A.
$$0.425\,g/c{m^3}$$
B.
$$8.5\,g/c{m^3}$$
C.
$$4.25\,g/c{m^3}$$
D.
$$82.5\,g/c{m^3}$$
Answer :
$$8.5\,g/c{m^3}$$
7. In $$NaCl$$ structure,
A.
all octahedral and tetrahedral sites are occupied
B.
only octahedral sites are occupied
C.
only tetrahedral sites are occupied
D.
neither octahedral nor tetrahedral sites are occupied.
Answer :
only octahedral sites are occupied
8. Copper crystallises in $$fcc$$ with a unit cell length of $$361 pm.$$ What is the radius of copper atom?
A.
$$127 pm$$
B.
$$157 pm$$
C.
$$181 pm$$
D.
$$108 pm$$
Answer :
$$127 pm$$
9. First three nearest neighbour distances for body centred cubic lattice are respectively :
A.
$$\sqrt 2 a,a,\sqrt 3 a$$
B.
$$\frac{a}{{\sqrt 2 }},a,\sqrt 3 a$$
C.
$$\frac{{\sqrt 3 a}}{2},a,\sqrt 2 a$$
D.
$$\frac{{\sqrt 3 a}}{2},a,\sqrt 3 a$$
Answer :
$$\frac{{\sqrt 3 a}}{2},a,\sqrt 2 a$$
10. A metal $$X$$ crystallises in a face-centred cubic arrangement with the edge length $$862\,pm.$$ What is the shortest separation of any two nuclei of the atom ?
A.
406$$\,pm$$
B.
707$$\,pm$$
C.
862$$\,pm$$
D.
609.6$$\,pm$$
Answer :
609.6$$\,pm$$