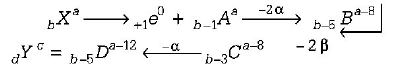1. $$Carbon-14$$ dating method is based on the fact that
A.
$$carbon-14$$ fraction is same in all objects
B.
$$carbon-14$$ is highly insoluble
C.
ratio of $$carbon-14$$ and $$carbon-12$$ is constant
D.
All of the above
Answer :
ratio of $$carbon-14$$ and $$carbon-12$$ is constant
2. In the following radioactive decay, $$_{92}{X^{232}}{ \to _{89}}{Y^{220}},$$ how many $$\alpha $$ and $$\beta $$ -particles are ejected from $$X$$ to $$Y?$$
A.
$$3\,\alpha \,{\text{and}}\,2\beta $$
B.
$$5\alpha \,{\text{and}}\,3\beta $$
C.
$$3\alpha \,{\text{and}}\,3\beta $$
D.
$$5\alpha \,{\text{and}}\,5\beta $$
Answer :
$$3\alpha \,{\text{and}}\,3\beta $$
3. Number of neutrons in a parent nucleus $$X,$$ which gives $$_7{N^{14}}$$ nucleus after two successive $$\beta $$ - emissions would be
A.
9
B.
6
C.
7
D.
8
Answer :
9
4. A nuclide of an alkaline earth metal undergoes radioactive decay by emission of three $$\alpha $$ - particles in succession. The group of the periodic table to which the resulting daughter element would belong is
A.
group 14
B.
group 16
C.
group 4
D.
group 6
Answer :
group 14
5. In a radioactive decay, an emitted electron comes from
A.
the nucleus of atom
B.
the orbit with principal quantum number 1
C.
the inner orbital of the atom
D.
the outermost orbit of the atom
Answer :
the nucleus of atom
6. If $$_b{X^a}$$ species emit firstly a positron, then two $$\alpha $$ and two $$\beta $$ and in last one $$\alpha $$ is also emitted and finally convert in $$_d{Y^c}$$ species, so correct the relation is
A.
$$a = c + 12,\,d = b - 5$$
B.
$$a = c - 8,\,d = b - 1$$
C.
$$a = c - 6,\,d = b - 0$$
D.
$$a = c - 4,\,d = b - 2$$
Answer :
$$a = c + 12,\,d = b - 5$$
7. Sulphur $$ = 35\left( {34.96903\,u} \right)$$ emits a $$\beta $$ - particle but no $$\gamma - ray.$$ The product is chlorine $$ = 35\,\left( {34.96885\,u} \right).$$ The maximum energy emitted by the $$\beta $$ - particle is
A.
16.758$$\,MeV$$
B.
1.6758$$\,MeV$$
C.
0.16758$$\,MeV$$
D.
0.016758$$\,MeV$$
Answer :
0.16758$$\,MeV$$
8. Half-life for radioactive $$^{14}C$$ is $$5760$$ $$yr.$$ In how many years, $$200$$ $$mg$$ of $$^{14}C$$ will be reduced to $$25$$ $$mg?$$
A.
5760$$\,yr$$
B.
11520$$\,yr$$
C.
17280$$\,yr$$
D.
23040$$\,yr$$
Answer :
17280$$\,yr$$
9. The half-life of $$_6{C^{14}},\left( {\lambda = 2.31 \times {{10}^{ - 4}}\,{\text{per}}\,{\text{year}}} \right)$$ is
A.
$$2 \times {10^2}\,yr$$
B.
$$3 \times {10^3}\,yr$$
C.
$$3.3 \times {10^4}\,yr$$
D.
$$4 \times {10^3}\,yr$$
Answer :
$$3 \times {10^3}\,yr$$
10. The half-life of a radioactive isotope is $$3 h.$$ If the initial mass of the isotope was $$300$$ $$g,$$ the mass which remained undecayed after $$18$$ $$h$$ would be
A.
4.68$$\,g$$
B.
2.34$$\,g$$
C.
1.17$$\,g$$
D.
9.36$$\,g$$
Answer :
4.68$$\,g$$