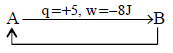1. The enthalpy of fusion of water is $$1.435\,kcal/mol.$$ The molar entropy change for the melting of ice at $${0^ \circ }C$$ is
A.
$$10.52\,cal/mol\,K$$
B.
$$21.04\,cal/mol\,K$$
C.
$$5.260\,cal/mol\,K$$
D.
$$0.526\,cal/mol\,K$$
Answer :
$$5.260\,cal/mol\,K$$
2. Which of the following statements is correct for the spontaneous absorption of a gas?
A.
$$\Delta S$$ is negative and therefore, $$\Delta H$$ should be highly positive
B.
$$\Delta S$$ is negative and therefore, $$\Delta H$$ should be highly negative
C.
$$\Delta S$$ is positive and therefore, $$\Delta H$$ should be negative
D.
$$\Delta S$$ is positive and therefore, $$\Delta H$$ should also be highly positive
Answer :
$$\Delta S$$ is negative and therefore, $$\Delta H$$ should be highly negative
3. If the bond energies of $$H-H,$$ $$Br-Br$$ and $$H-Br$$ are $$433, 192$$ and $$364\,kJ\,mo{l^{ - 1}}$$ respectively, then $$\Delta {H^ \circ }$$ for the reaction $${H_2}\left( g \right) + B{r_2}\left( g \right) \to 2HBr\left( g \right)$$ is
A.
$$ - 261\,kJ$$
B.
$$ + 103\,kJ$$
C.
$$ + 261\,kJ$$
D.
$$ - 103\,kJ$$
Answer :
$$ - 103\,kJ$$
4. Following reaction occurrs in an automobile $$2{C_8}{H_{18}}\left( g \right) + 25{O_2}\left( g \right) \to 16C{O_2}\left( g \right) + 18{H_2}O\left( g \right).$$ The sign of $$\Delta H,\Delta S$$ and $$\Delta G$$ would be
A.
$$ + , - , + $$
B.
$$ - , + , - $$
C.
$$ - , + , + $$
D.
$$ + , + , - $$
Answer :
$$ - , + , - $$
5. In a reaction $$P + Q \to R + S,$$ there is no change in entropy. Enthalpy change for the reaction $$\left( {\Delta H} \right)$$ is $$12\,kJ\,mo{l^{ - 1}}.$$ Under what conditions, reaction will have negative value of free energy change ?
A.
If $${\Delta H}$$ is positive.
B.
If $${\Delta H}$$ is negative.
C.
If $${\Delta H}$$ is $$24\,kJ\,mo{l^{ - 1}}.$$
D.
If temperature of reaction is high.
Answer :
If $${\Delta H}$$ is negative.
6. Which one of the following statements is false?
A.
Work is a state function.
B.
Temperature is a state function.
C.
Change in the state is completely defined when the initial and final states are specified.
D.
Work appears at the boundary of the system.
Answer :
Work is a state function.
7. A gas undergoes change from state $$A$$ to state $$B.$$ In this process, the heat absorbed and work done by the gas is $$5\,J$$ and $$8\,J,$$ respectively. Now gas is brought back to $$A$$ by another process during which $$3\,J$$ of heat is evolved. In this reverse process of $$B$$ to $$A$$ :
A.
$$10\,J$$ of the work will be done by the gas.
B.
$$6\,J$$ of the work will be done by the gas.
C.
$$10\,J$$ of the work will be done by the surrounding on gas.
D.
$$6\,J$$ of the work will be done by the surrounding on gas.
Answer :
$$6\,J$$ of the work will be done by the surrounding on gas.
8. If the bond dissociation energies of $$XY,{X_2}$$ and $${Y_2}$$ ( all diatomic molecules ) are in the ratio of 1 : 1 : 0.5 and $$\Delta {H_f}$$ for the formation of $$XY$$ is $$ - 200\,kJ\,mo{l^{ - 1}}.$$ The bond dissociation energy of $${X_2}$$ will be
A.
$$400\,kJ\,mo{l^{ - 1}}$$
B.
$$800\,kJ\,mo{l^{ - 1}}$$
C.
$$200\,k{J^{ - 1}}$$
D.
$$100\,k{J^{ - 1}}$$
Answer :
9. Which is the correct relationship between $$\Delta {G^ \circ }$$ and equilibrium constant $${K_p}?$$
A.
$${K_p} = - RT\,\,\log \,\,\Delta {G^ \circ }$$
B.
$${K_p} = {\left[ {\frac{e}{{RT}}} \right]^{\Delta {G^ \circ }}}$$
C.
$${K_p} = - \frac{{\Delta {G^ \circ }}}{{RT}}$$
D.
$${K_p} = {e^{ - \,\frac{{\Delta {G^ \circ }}}{{RT}}}}$$
Answer :
$${K_p} = {e^{ - \,\frac{{\Delta {G^ \circ }}}{{RT}}}}$$
10.
Two reactions are given below :
$$\eqalign{
& \left( {\text{i}} \right)C{O_{\left( g \right)}} + \frac{1}{2}{O_{2\left( g \right)}} \to C{O_{2\left( g \right)}} \cr
& \left( {{\text{ii}}} \right)A{g_2}{O_{\left( s \right)}} \to 2A{g_{\left( s \right)}} + \frac{1}{2}{O_{2\left( g \right)}} \cr} $$
Which of the following statements is true ?
A.
For $$\left( {\text{i}} \right)\Delta H < \Delta U$$ and for $$\left( {{\text{ii}}} \right)\Delta H > \Delta U$$
B.
For $$\left( {\text{i}} \right)\Delta H > \Delta U$$ and for $$\left( {{\text{ii}}} \right)\Delta H < \Delta U$$
C.
For both $$\left( {\text{i}} \right)$$ and $$\left( {{\text{ii}}} \right)\Delta H > \Delta U$$
D.
For both $$\left( {\text{i}} \right)$$ and $$\left( {{\text{ii}}} \right)\Delta H < \Delta U$$
Answer :
For $$\left( {\text{i}} \right)\Delta H < \Delta U$$ and for $$\left( {{\text{ii}}} \right)\Delta H > \Delta U$$