1.
Reaction$$Ba{O_2}\left( s \right) \rightleftharpoons BaO\left( s \right) + {O_2}\left( g \right),$$ $$\Delta H = + ve$$
In equilibrium condition, pressure of $${O_2}$$ depends on
A.
increased mass of $$Ba{O_2}$$
B.
increased mass of $$BaO$$
C.
increased temperature of equilibrium
D.
increased mass of $$Ba{O_2}$$ and $$BaO$$ both
Answer :
increased temperature of equilibrium
2. The equilibrium constant at $$298 K$$ for a reaction $$A + B \rightleftharpoons C + D$$ is 100. If the initial concentration of all the four species were $$1 M$$ each, then equilibrium concentration of $$D\left( {{\text{in}}\,{\text{mol}}\,{L^{ - 1}}} \right)$$ will be :
A.
1.818
B.
1.182
C.
0.182
D.
0.818
Answer :
1.818
3.
The value of equilibrium constant of the reaction, $$HI\left( g \right) \rightleftharpoons \frac{1}{2}{H_2}\left( g \right) + \frac{1}{2}{I_2}\left( g \right)$$ is 8.0.
The equilibrium constant of the reaction, $${H_2}\left( g \right) + {I_2}\left( g \right) \rightleftharpoons 2HI\left( g \right)$$ will be
A.
$$\frac{1}{{16}}$$
B.
$$\frac{1}{{64}}$$
C.
$$16$$
D.
$$\frac{1}{8}$$
Answer :
$$\frac{1}{{64}}$$
4. The expression for equilibrium constant, $${K_c}$$ for the following reaction is $$2Cu{\left( {N{O_3}} \right)_{2\left( s \right)}} \rightleftharpoons $$ $$2Cu{O_{\left( s \right)}} + 4N{O_{2\left( g \right)}} + {O_{2\left( g \right)}}$$
A.
$${K_c} = \frac{{{{\left[ {Cu{O_{\left( s \right)}}} \right]}^2}{{\left[ {N{O_{2\left( g \right)}}} \right]}^4}\left[ {{O_{2\left( g \right)}}} \right]}}{{{{\left[ {Cu{{\left( {N{O_3}} \right)}_{2\left( s \right)}}} \right]}^2}}}$$
B.
$${K_c} = \frac{{{{\left[ {N{O_{2\left( g \right)}}} \right]}^4}\left[ {{O_{2\left( g \right)}}} \right]}}{{{{\left[ {Cu{{\left( {N{O_3}} \right)}_{2\left( s \right)}}} \right]}^2}}}$$
C.
$${K_c} = {\left[ {N{O_{2\left( g \right)}}} \right]^4}\left[ {{O_{2\left( g \right)}}} \right]$$
D.
$${K_c} = \frac{{{{\left[ {Cu{O_{\left( s \right)}}} \right]}^2}}}{{{{\left[ {Cu{{\left( {N{O_3}} \right)}_{2\left( s \right)}}} \right]}^2}}}$$
Answer :
$${K_c} = {\left[ {N{O_{2\left( g \right)}}} \right]^4}\left[ {{O_{2\left( g \right)}}} \right]$$
5. $$5\,moles$$ of $$PC{l_5}$$ are heated in a closed vessel of 5 litre capacity. At equilibrium $$40\% $$ of $$PC{l_5}$$ is found to be dissociated. What is the value of $${K_c}?$$
A.
0.266$$\,M$$
B.
0.133$$\,M$$
C.
2.5$$\,M$$
D.
0.20$$\,M$$
Answer :
0.266$$\,M$$
6. For the reaction : $$2Ba{O_2}\left( s \right) \rightleftharpoons 2BaO\left( s \right) + {O_2}\left( g \right);$$ $$\Delta H = + ve.$$ In equilibrium condition, pressure of $${O_2}$$ is dependent on
A.
mass of $$Ba{O_2}$$
B.
mass of $$BaO$$
C.
temperature of equilibrium
D.
mass of $$Ba{O_2}$$ and $$BaO$$ both
Answer :
temperature of equilibrium
7.
The $$\% $$ yield of ammonia as a function of time in the reaction $${N_{2\left( g \right)}} + 3{H_{2\left( g \right)}} \rightleftharpoons 2N{H_{3\left( g \right)}},$$ $$\Delta H < 0$$ at $$\left( {P,{T_1}} \right)$$ is given below.
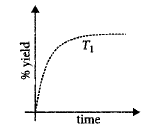
If this reaction is conducted at $$\left( {P,{T_2}} \right),$$ with $${T_2} > {T_1},$$ the $$\% $$ yield of ammonia as a function of time is represented by
A.
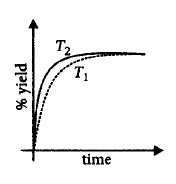

B.
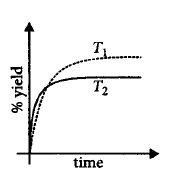

C.
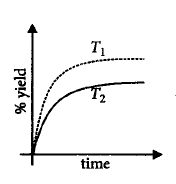

D.
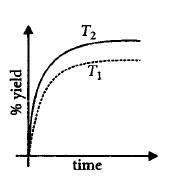

Answer :


8. Which one of the following information can be obtained on the basis of Le-Chatelier’s principle?
A.
Dissociation constant of a weak acid
B.
Entropy change in a reaction
C.
Equilibrium constant of a chemical reaction
D.
Shift in equilibrium position on changing value of a constant
Answer :
Shift in equilibrium position on changing value of a constant
9. For the reaction equilibrium $${N_2}{O_4}\left( g \right) \rightleftharpoons 2\,N{O_2}\left( g \right)$$ the concentrations of $${N_2}{O_4}$$ and $$N{O_2}$$ at equilibrium are $$4.8 \times {10^{ - 2}}$$ and $$1.2 \times {10^{ - 2}}mol\,{L^{ - 1}}$$ respectively. The value of $${K_c}$$ for the reaction is
A.
$$3 \times {10^{ - 1}}mol\,{L^{ - 1}}$$
B.
$$3 \times {10^{ - 3}}mol\,{L^{ - 1}}$$
C.
$$3 \times {10^3}mol\,{L^{ - 1}}$$
D.
$$3.3 \times {10^2}mol\,{L^{ - 1}}$$
Answer :
$$3 \times {10^{ - 3}}mol\,{L^{ - 1}}$$
10.
For the reaction $$:2N{O_{2\left( g \right)}} \rightleftharpoons 2N{O_{\left( g \right)}} + {O_{2\left( g \right)}},$$
$$\left( {{K_c} = 1.8 \times {{10}^{ - 6}}\,{\text{at}}\,{{184}^ \circ }C} \right)\left( {R = 0.0831\,kJ/\left( {mol.\,K} \right)} \right)$$
When $${K_p}$$ and $${K_c}$$ are compared at $${{{184}^ \circ }C}$$ it is found that
A.
Whether $${K_p}$$ is greater than, less than or equal to $${K_c}$$ depends upon the total gas pressure
B.
$${K_p} = {K_c}$$
C.
$${K_p}$$ is less than $${K_c}$$
D.
$${K_p}$$ is greater than $${K_c}$$
Answer :
$${K_p}$$ is greater than $${K_c}$$