1.
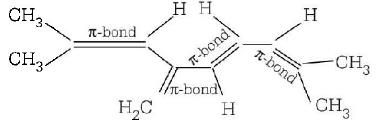
The total number of $$\pi $$ - bond electrons in the following structure is
A.
4
B.
8
C.
12
D.
16
Answer :
8
2. Hyperconjugation is
A.
$$\sigma - \pi $$ conjugation
B.
noticed due to delocalisation of $$\sigma $$ and $$\pi $$ bonds
C.
no bond resonance
D.
all the above
Answer :
all the above
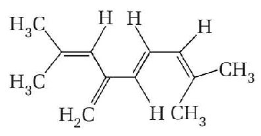
3.
Decreasing order of stability of following alkenes is
\[\begin{align}
& \left( \text{i} \right)C{{H}_{3}}-CH=C{{H}_{2}} \\
& \left( \text{ii} \right)C{{H}_{3}}-CH=CH-C{{H}_{3}} \\
& \left( \text{iii} \right)C{{H}_{3}}\underset{\begin{smallmatrix}
| \\
\,\,\,C{{H}_{3}}
\end{smallmatrix}}{\mathop{-\,\,C=}}\,CH-C{{H}_{3}} \\
& \left( \text{iv} \right)C{{H}_{3}}\,\,\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{-\,\,C\,=}}\,\overset{\begin{smallmatrix}
\,\,\,\,C{{H}_{3}} \\
|
\end{smallmatrix}}{\mathop{\,\,\,C\,\,-}}\,\,\,C{{H}_{3}} \\
\end{align}\]
A.
(i) > (ii) > (iii) > (iv)
B.
(iv) > (iii) > (ii) > (i)
C.
(iii) > (ii) > (i) > (iv)
D.
(ii) > (iii) > (iv) > (i)
Answer :
(iv) > (iii) > (ii) > (i)
4.
The order of decreasing stability of the following carbanions is
$$\eqalign{
& \left( {\text{i}} \right){\left( {C{H_3}} \right)_3}{C^ - } \cr
& \left( {{\text{ii}}} \right){\left( {C{H_3}} \right)_2}C{H^ - } \cr
& \left( {{\text{iii}}} \right)C{H_3}CH_2^ - \cr
& \left( {{\text{iv}}} \right){C_6}{H_5}CH_2^ - \cr} $$
A.
(i) > (ii) > (iii) > (iv)
B.
(iv) > (iii) > (ii) > (i)
C.
(iv) > (i) > (ii) > (iii)
D.
(iii) > (ii) > (i) > (iv)
Answer :
(iv) > (iii) > (ii) > (i)
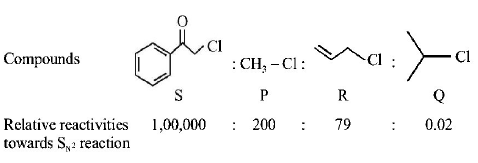
5.
Kl in acetone, undergoes $${S_N}2$$ reaction with each of $$P, Q, R$$ and $$S.$$ The rates of the reaction vary as
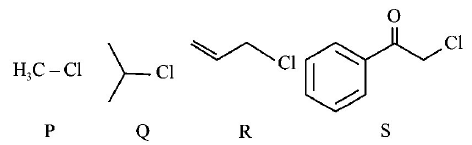
A.
$$P > Q > R > S$$
B.
$$S > P > R > Q$$
C.
$$P > R > Q > S$$
D.
$$R > P > S > Q$$
Answer :
$$S > P > R > Q$$
6. Heterolysis of a carbon-chlorine bond produces
A.
two free radicals
B.
two carbocations
C.
one cation and one anion
D.
two carbanions
Answer :
one cation and one anion
7. Point out the incorrect statement about resonance.
A.
Resonance structures should have equal energy.
B.
In resonance structures, the constituent atoms must be in the same position.
C.
In resonance structures, there should not be same number of electron pairs.
D.
Resonance structures should differ only in the location of electrons around the constituent atoms.
Answer :
In resonance structures, there should not be same number of electron pairs.
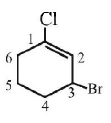
8.
The IUPAC name of the compound shown below is :
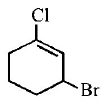
A.
3 - bromo - 1- chlorocyclohexene
B.
1 - bromo - 3 - chlorocyclohexene
C.
2 - bromo - 6 - chlorocyclohex - 1 - ene
D.
6 - bromo - 2 - chlorocyclohexene
Answer :
3 - bromo - 1- chlorocyclohexene
9. Dipole moment is shown by
A.
1, 2-dichlorobenzene
B.
$$trans$$ 2, 3-dichloro-2-butene
C.
1, 4-chlorobenzene
D.
$$trans$$ -1, 2-dinitroethene
Answer :
1, 2-dichlorobenzene
10.
The IUPAC name of the compound 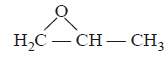 is
is
A.
1, 2-Propoxide
B.
Propylene oxide
C.
1, 2-Oxo propane
D.
1, 2-Epoxy propane
Answer :
1, 2-Epoxy propane