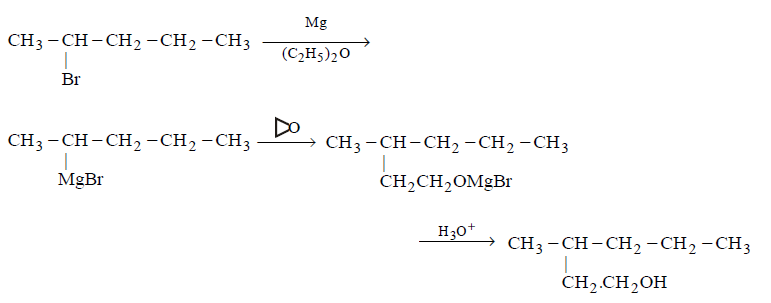1. Reagent used to convert allyl alcohol to acrolein is
A.
$$Mn{O_2}$$
B.
$${H_2}{O_2}$$
C.
$$Os{O_4}$$
D.
$$KMn{O_4}$$
Answer :
$$Mn{O_2}$$
2. What would be the reactant and reagent used to obtain 2, 4-dimethylpentan-3-ol?
A.
Propanal and propyl magnesium bromide
B.
3-Methylbutanal and 2-methyl magnesium iodide
C.
2, 2-Dimethylpropanone and methyl magnesium iodide
D.
2-Methylpropanal and isopropyl magnesium iodide
Answer :
2-Methylpropanal and isopropyl magnesium iodide
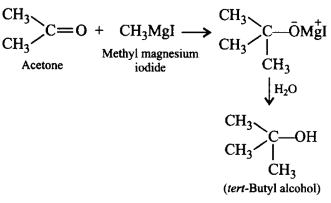
3. Tertiary butyl alcohol can be prepared by the reaction of
A.
acetaldehyde and ethyl magnesium iodide
B.
acetone and methyl magnesium iodide
C.
formaldehyde and propyl magnesium iodide
D.
butanone and methyl magnesium iodide
Answer :
acetone and methyl magnesium iodide
4. Which of the following alcohols gives the best yield of dialkyl ether on being heated with a trace of sulphuric acid?
A.
2-Pentanol
B.
2-Methyl-2-butanol
C.
1-Pentanol
D.
2-Propanol
Answer :
1-Pentanol
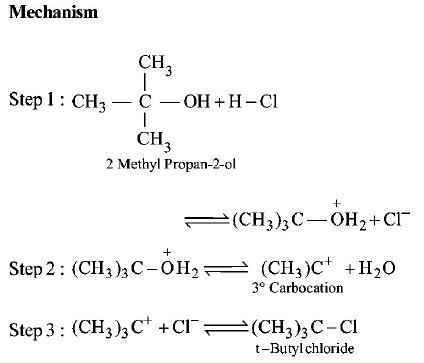
5. From amongst the following alcohols the one that would react fastest with conc. $$HCl$$ and anhydrous $$ZnC{l_2},$$ is
A.
2 - Butanol
B.
2 - Methylpropan - 2 - $$ol$$
C.
2 - Methylpropanol
D.
1 - Butanol
Answer :
2 - Methylpropan - 2 - $$ol$$
6.
A primary alcohol, $${C_3}{H_8}O\left( A \right)$$ on heating with sulphuric acid undergo dehydration to give an alkene, $$B.$$ $$B$$ when reacted with $$HCl$$ gave $$C,$$ which on treatment with aqueous $$KOH$$ gives compound $$D\left( {{C_3}{H_8}O} \right).$$
$$A$$ and $$D$$ are
A.
functional isomers
B.
position isomers
C.
chain isomers
D.
stereoisomers
Answer :
position isomers
7. Which of the following compounds is a polyhydric alcohol?
A.
$${C_2}{H_6}O$$
B.
$${C_2}{H_4}O$$
C.
$${C_3}{H_8}{O_3}$$
D.
$${C_4}{H_{10}}O$$
Answer :
$${C_3}{H_8}{O_3}$$
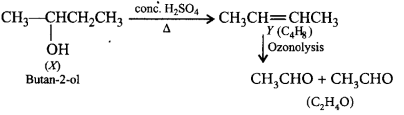
8. An alcohol $$X$$ when treated with hot conc. $${H_2}S{O_4}$$ gave an alkene $$ Y$$ with formula $${C_4}{H_8}.$$ This alkene on ozonolysis gives single product with molecular formula $${C_2}{H_4}O.$$ The alcohol is
A.
butan-1-ol
B.
butan-2-ol
C.
2-methylpropan-1-ol
D.
2, 2-dimethylbutan-1-ol
Answer :
butan-2-ol
9. Which of the following synthesis gives 3-methyl-1- hexanol ?
A.
2 - bromohexane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,{{H}_{2}}C=O}\]
B.
2 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\] 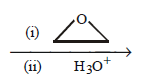
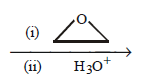
C.
3 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,C{{H}_{3}}CH=O}\]
D.
1 - bromobutane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\,\,\,\xrightarrow[\left( ii \right)\,{{H}_{3}}{{O}^{+}}]{\left( i \right)\,C{{H}_{3}}COC{{H}_{3}}}\]
Answer :
2 - bromopentane \[\xrightarrow[{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}]{Mg}\] 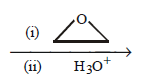
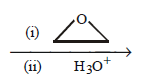
10. If a particular ether contains $$60\% $$ carbon and $$13.3\% $$ hydrogen, its formula could be
A.
$${C_2}{H_6}O$$
B.
$${C_4}{H_{10}}O$$
C.
$${C_5}{H_{12}}O$$
D.
$${C_3}{H_8}O$$
Answer :
$${C_3}{H_8}O$$