1. Magnesium powder burns in air to give:
A.
$$Mg{\left( {N{O_3}} \right)_2}\,{\text{and}}\,M{g_3}{N_2}$$
B.
$$MgO\,{\text{and}}\,M{g_3}{N_2}$$
C.
$$MgO\,{\text{only}}$$
D.
$$MgO\,{\text{and}}\,Mg{\left( {N{O_3}} \right)_2}$$
Answer :
$$MgO\,{\text{and}}\,M{g_3}{N_2}$$
2.
All alkali halides are soluble in water except $$LiF.$$ The low solubility of $$LiF$$ in water is due to its $$\underline {\left( {\text{i}} \right)} $$ the low solubility of $$CsI$$ is due to $$\underline {\left( {{\text{ii}}} \right)} .$$ $$LiF$$ is soluble in $$\underline {\left( {{\text{iii}}} \right)} $$ solvents.
(i)
(ii)
(iii)
(a)
low lattice enthalpy
large hydration enthalpy
polar solvents
(b)
high lattice enthalpy
smaller hydration enthalpy
non - polar solvents
(c)
high hydration enthalpy
high lattice enthalpy
non - polar solvents
(d)
smaller hydration enthalpy
high lattice enthalpy
polar solvents
A.
(a)
B.
(b)
C.
(c)
D.
(d)
Answer :
(b)
3.
When alkaline earth metals dissolve in ammonia, they form coloured solution like alkali metals. Which of the following observations regarding the reaction are correct?
(i) Dilute solutions are bright blue in colour due to solvated electrons.
(ii) These solutions decompose to form amides and hydrogen.
(iii) From this solution the ammoniates $${\left[ {M{{\left( {N{H_3}} \right)}_6}} \right]^{2 + }}$$ can be recovered by evaporation.
A.
Only (i) and (ii)
B.
(i), (ii) and (iii)
C.
Only (ii) and (iii)
D.
Only (i)
Answer :
(i), (ii) and (iii)
4.
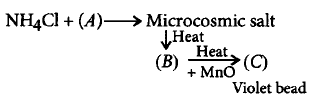
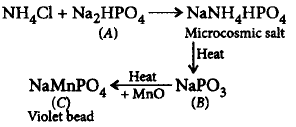
$$(A), (B)$$ and $$(C)$$ respectively are
A.
$$N{a_3}P{O_4},NaP{O_3},{\left( {Mn} \right)_3}{\left( {P{O_4}} \right)_2}$$
B.
$$N{a_2}HP{O_4},N{a_3}P{O_4},M{n_3}{\left( {P{O_4}} \right)_2}$$
C.
$$N{a_2}HP{O_4},NaP{O_3},Mn{\left( {P{O_3}} \right)_2}$$
D.
$$N{a_2}HP{O_4},NaP{O_3},NaMnP{O_4}$$
Answer :
$$N{a_2}HP{O_4},NaP{O_3},NaMnP{O_4}$$
5. Which is the characteristic flame colouration of $$Li?$$
A.
Yellow
B.
Violet
C.
Blue
D.
Crimson red
Answer :
Crimson red
6. Which one of the following processes will produce hard water?
A.
Saturation of water with $$MgC{O_3}$$
B.
Saturation of water with $$CaS{O_4}$$
C.
Addition of $$N{a_2}S{O_4}$$ to water
D.
Saturation of water with $$CaC{O_3}$$
Answer :
Saturation of water with $$CaS{O_4}$$
7. The solubility of alkali metal salts in water is due to the fact that the cations get hydrated by water molecules. The degree of hydration depends upon the size of the cation. If the trend of relative ionic radii is $$C{s^ + } > R{b^ + } > {K^ + } > N{a^ + } > L{i^ + }.$$ What is the relative degree of hydration?
A.
$$Cs_{\left( {aq} \right)}^ + > Rb_{\left( {aq} \right)}^ + > K_{\left( {aq} \right)}^ + > Na_{\left( {aq} \right)}^ + > Li_{\left( {aq} \right)}^ + $$
B.
$$Li_{\left( {aq} \right)}^ + > Na_{\left( {aq} \right)}^ + > K_{\left( {aq} \right)}^ + > Rb_{\left( {aq} \right)}^ + > Cs_{\left( {aq} \right)}^ + $$
C.
$$Na_{\left( {aq} \right)}^ + > K_{\left( {aq} \right)}^ + > Rb_{\left( {aq} \right)}^ + > Cs_{\left( {aq} \right)}^ + > Li_{\left( {aq} \right)}^ + $$
D.
$$Cs_{\left( {aq} \right)}^ + > Na_{\left( {aq} \right)}^ + > Li_{\left( {aq} \right)}^ + > K_{\left( {aq} \right)}^ + > Rb_{\left( {aq} \right)}^ + $$
Answer :
$$Li_{\left( {aq} \right)}^ + > Na_{\left( {aq} \right)}^ + > K_{\left( {aq} \right)}^ + > Rb_{\left( {aq} \right)}^ + > Cs_{\left( {aq} \right)}^ + $$
8. The mobilities of the alkali metal ions in aqueous solution are $$L{i^ + } < N{a^ + } < {K^ + } < R{b^ + } < C{s^ + }$$ because
A.
greater is the degree of hydration, lesser is the mobility in aqueous medium
B.
larger the size of cation, greater is the mobility in aqueous medium
C.
larger the size of cation, lesser is the mobility of ions in aqueous medium
D.
lesser the degree of hydration, lesser is the mobility of ions in aqueous medium
Answer :
greater is the degree of hydration, lesser is the mobility in aqueous medium
9. In context with beryllium, which one of the following statements is incorrect ?
A.
It is rendered passive by nitric acid
B.
It forms $$B{e_2}C$$
C.
Its salts rarely hydrolyse
D.
Its hydride is electron-deficient and polymeric
Answer :
Its salts rarely hydrolyse
10.
Identify $$W, X, Y$$ and $$Z$$ respectively in the given reactions.
\[CaC{{O}_{3}}\xrightarrow{\Delta }W+X\]
$$\eqalign{
& W + {H_2}O \to Y \cr
& Y + Z \to NaOH + CaC{O_3} \cr} $$
$$W$$
$$X$$
$$Y$$
$$Z$$
(a)
$$CaO$$
$$C{O_2}$$
$$CaC{O_3}$$
$$N{a_2}C{O_3}$$
(b)
$$C{O_2}$$
$$Ca{\left( {OH} \right)_2}$$
$$Ca{\left( {HCO} \right)_3}$$
$$NaHC{O_3}$$
(c)
$$CaO$$
$$C{O_2}$$
$$Ca{\left( {OH} \right)_2}$$
$$N{a_2}C{O_3}$$
(d)
$$C{O_2}$$
$$CaO$$
$${H_2}C{O_3}$$
$$N{a_2}C{O_3}$$
A.
(a)
B.
(b)
C.
(c)
D.
(d)
Answer :
(c)