1. Potassium chromate solution is added to an aqueous solution of a metal chloride. The precipitate thus obtained is insoluble in acetic acid. When precipitate is subjected to flame test the colour of the flame is
A.
lilac
B.
apple green
C.
crimson red
D.
golden yellow
Answer :
apple green
2. A gas $$“X”$$ is passed through water to form a saturated solution. The aqueous solutions on treatment with the $$AgN{O_3}$$ gives a white preciptate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colourless gas $$“Y”.$$ Identify $$'X'$$ and $$'Y'.$$
A.
$$X = C{O_2},Y = C{l_2}$$
B.
$$X = C{l_2},Y = C{O_2}$$
C.
$$X = C{l_2},Y = {H_2}$$
D.
$$X = {H_2},Y = C{l_2}$$
Answer :
$$X = C{l_2},Y = {H_2}$$
3. Sodium carbonate cannot be used in place of $${\left( {N{H_4}} \right)_2}C{O_3}$$ for the identification of $$C{a^{2 + }},B{a^{2 + }}$$ and $$S{r^{2 + }}ions$$ ( in group $$V$$ ) during mixture analysis because :
A.
$$M{g^{2 + }}\,ions$$ will also be precipitated.
B.
Concentration of $$CO_3^{2 - }\,ions$$ is very low.
C.
Sodium ions will react with acid radicals.
D.
$$N{a^ + }\,ions$$ will interfere with the detection of $$C{a^{2 + }},B{a^{2 + }},S{r^{2 + }}\,ions.$$
Answer :
$$M{g^{2 + }}\,ions$$ will also be precipitated.
4. The sodium extract prepared from sulphanilic acid, contains $$SC{N^ - }.$$ It gives blood red colouration with
A.
a mixture of $$N{a_2}S$$ and $$C{S_2}$$
B.
$$FeC{l_3}$$
C.
$$FeS{O_4}$$
D.
$$N{a_2}S{O_3}$$
Answer :
$$FeC{l_3}$$
5. A solution when diluted with $${H_2}O$$ and boiled, gives a white precipitate. On addition of excess $$N{H_4}Cl/N{H_4}OH,$$ the volume of precipitate decreases leaving behind a white gelatinous precipitate. Identify the precipitate which disolves in $$N{H_4}OH/N{H_4}Cl$$
A.
$$Al{\left( {OH} \right)_3}$$
B.
$$Zn{\left( {OH} \right)_2}$$
C.
$$Ca{\left( {OH} \right)_2}$$
D.
$$Mg{\left( {OH} \right)_2}$$
Answer :
$$Zn{\left( {OH} \right)_2}$$
6. Prussian blue is formed when
A.
ferrous sulphate reacts with $$FeC{l_3}$$
B.
ferric sulphate reacts with $$N{a_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
C.
ferrous ammonium sulphate reacts with $$FeC{l_3}$$
D.
ammonium sulphate reacts with $$FeC{l_3}$$
Answer :
ferric sulphate reacts with $$N{a_4}\left[ {Fe{{\left( {CN} \right)}_6}} \right]$$
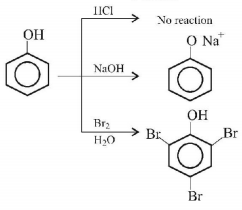
7.
The organic compound that gives following qualitative analysis is:

A.
.PNG)
.PNG)
B.
.PNG)
.PNG)
C.
.PNG)
.PNG)
D.
.PNG)
.PNG)
Answer :
.PNG)
.PNG)
8.
A substance on treatment with $$dil.{H_2}S{O_4}$$ liberates a colourless gas which produces
(I) turbidity with baryta water and
(II) turns acidified dichromate solution green.
The reaction indicates the presence of
A.
$$CO_3^{2 - }$$
B.
$${S^{2 - }}$$
C.
$$SO_3^{2 - }$$
D.
$$NO_3^ - $$
Answer :
$$SO_3^{2 - }$$
9. Which of the following statements is incorrect ?
A.
$$F{e^{2 + }}\,ion$$ also gives blood red colour with $$SC{N^ - }\,ion.$$
B.
$$F{e^{3 + }}\,ion$$ also gives blood red colour with $$SC{N^ - }\,ion.$$
C.
On passing $${H_2}S$$ into $$N{a_2}Zn{O_2}$$ solution a white ppt of $$ZnS$$ is formed.
D.
Cupric ion reacts with excess of ammonia solution to give deep blue colour of $${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}\,ion.$$
Answer :
$$F{e^{2 + }}\,ion$$ also gives blood red colour with $$SC{N^ - }\,ion.$$
10.
An aqueous solution contains $$H{g^{2 + }},Hg_2^{2 + },P{b^{2 + }}\,{\text{and}}\,C{d^{2 + }}.$$
The addition of $$HCl\left( {6N} \right)$$ will precipitate:
A.
$$H{g_2}C{l_2}\,{\text{only}}$$
B.
$$PbC{l_2}\,{\text{only}}$$
C.
$$PbC{l_2}\,{\text{and}}\,H{g_2}C{l_2}$$
D.
$$PbC{l_2}\,{\text{and}}\,HgC{l_2}$$
Answer :
$$PbC{l_2}\,{\text{and}}\,H{g_2}C{l_2}$$