1. Which of the following techniques is most suitable for purification of cyclohexanone from a mixture containing benzoic acid, isoamyl alcohol, cyclohexane and cyclohexanone?
A.
Crystallisation
B.
$$IR$$ spectroscopy
C.
Sublimation
D.
Evaporation
Answer :
$$IR$$ spectroscopy
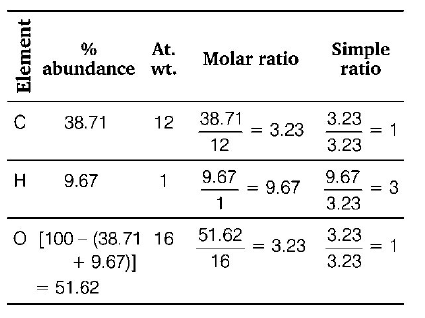
2. An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave $$C,38.71\% $$ and $$H,9.67\% .$$ The empirical formula of the compound would be
A.
$$C{H_3}O$$
B.
$$C{H_2}O$$
C.
$$CHO$$
D.
$$C{H_4}O$$
Answer :
$$C{H_3}O$$
3.
$$0.24\,g$$ of a volatile liquid on vaporization gives $$45\,ml$$ of vapours at $$NTP.$$ What will be the vapour density of the substance ?
$$\left( {{\text{Density of}}\,{\text{ }}{H_2} = 0.089{\text{ }}g{\text{ }}{L^{ - 1}}} \right)$$
A.
95.39
B.
39.95
C.
99.53
D.
59.93
Answer :
59.93
4. Which of the following compounds will be suitable for Kjeldahl’s method for nitrogen estimation?
A.

B.
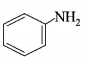

C.
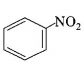

D.
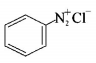

Answer :


5. In Duma’s method of estimation of nitrogen $$0.35\,g$$ of an organic compound gave $$55\,ml$$ of nitrogen collected at $$300\,K$$ temperature and $$175\,mm$$ pressure. The percentage composition of nitrogen in the compound would be ( Aqueous tension at $$300\,K = 15\,mm$$ )
A.
16.45
B.
17.45
C.
14.45
D.
15.45
Answer :
16.45
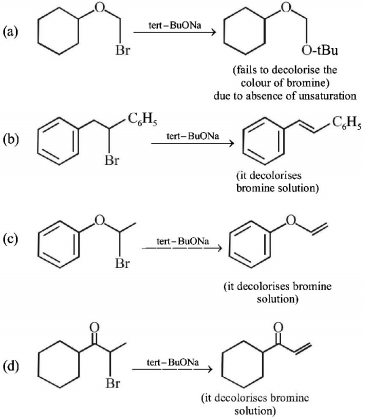
6. Which of the following , upon treatment with tert-BuONa followed by addition of bromine water, fails to decolourize the colour of bromine?
A.
.PNG)
.PNG)
B.
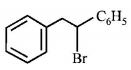

C.
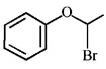

D.
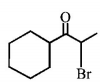

Answer :
.PNG)
.PNG)
7. $$0.5\,g$$ mixture of $${K_2}C{r_2}{O_7}$$ and $$KMn{O_4}$$ was treated with excess of $$KI$$ in acidic medium. $${I_2}$$ liberated required $$100\,c{m^3}$$ of $$0.15\,N\,N{a_2}{S_2}{O_3}$$ solution for titration. The percentage amount of $${K_2}C{r_2}{O_7}$$ in the mixture is
A.
85.36%
B.
14.64%
C.
58.63%
D.
26.14%
Answer :
14.64%
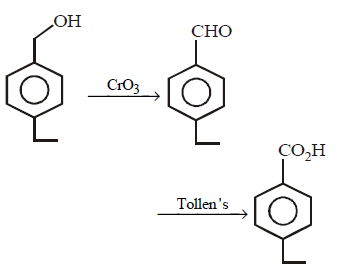
8.
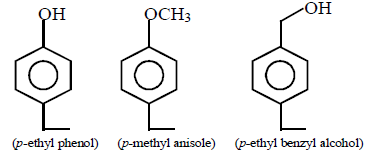
Above compounds can be differentiated by using the reagent :
A.
$$NaOH,$$ Tollen's reagent, $$FeC{l_3}$$
B.
$$Cr{O_3},$$ Tollen's reagent, $$FeC{l_3}$$
C.
Tollen's reagent, $$Cr{O_3},FeC{l_3}$$
D.
$$Na,$$ Tollen's reagent, $$FeC{l_3}$$
Answer :
$$Cr{O_3},$$ Tollen's reagent, $$FeC{l_3}$$
9. The Lassaigne’s extract is boiled with $$dil.\,HN{O_3}$$ before testing for halogens because
A.
silver halides are soluble in $$HN{O_3}$$
B.
$$N{a_2}S$$ and $$NaCN$$ are decomposed by $$HN{O_3}$$
C.
$$A{g_2}S$$ is soluble in $$HN{O_3}$$
D.
$$AgCN$$ is soluble in $$HN{O_3}$$
Answer :
$$N{a_2}S$$ and $$NaCN$$ are decomposed by $$HN{O_3}$$
10. In Duma’s method for estimation of nitrogen, $$0.25\,g$$ of an organic compound gave $$40\,mL$$ of nitrogen collected at $$300\,K$$ temperature and $$725\,mm$$ pressure. If the aqueous tension at $$300\,K$$ is $$25\,mm,$$ the percentage of nitrogen in the compound is
A.
17.36
B.
18.20
C.
16.76
D.
15.76
Answer :
16.76